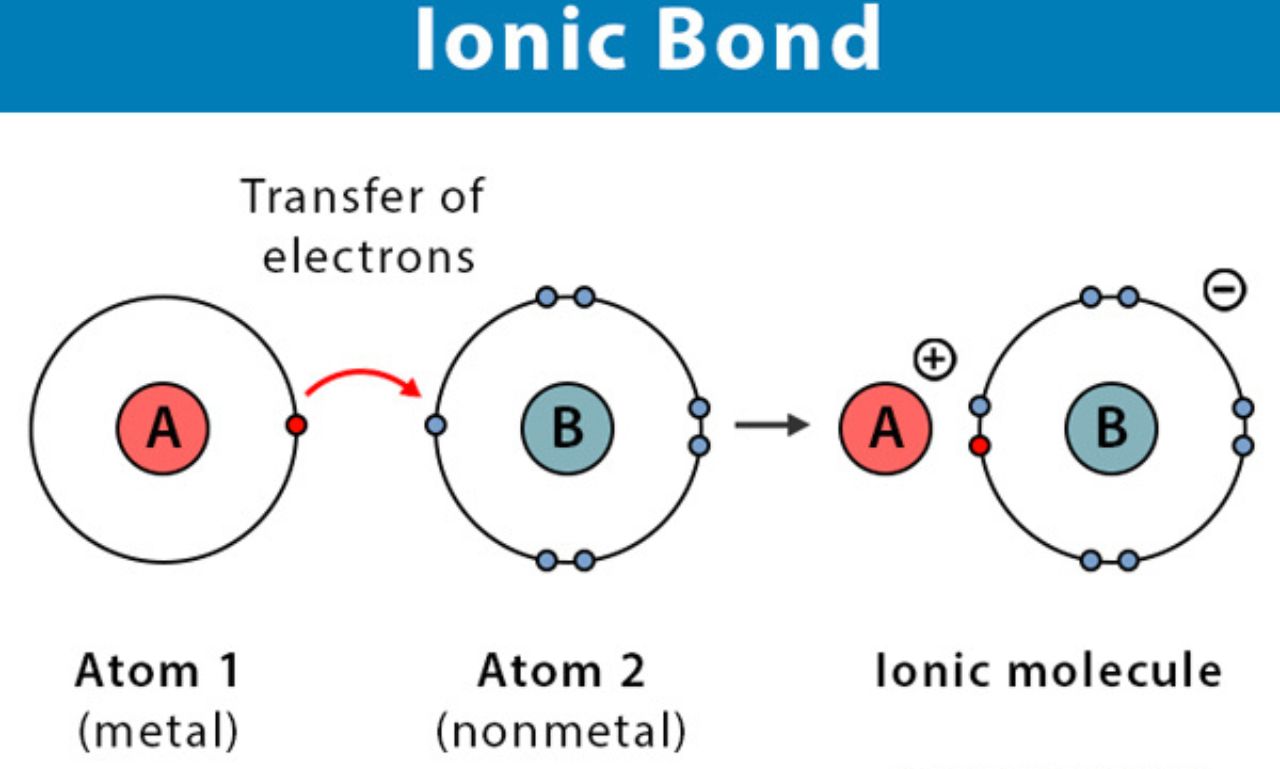
An ionic bond is one of the most fundamental types of chemical bonding. It occurs when atoms transfer electrons from one to another, creating positively and negatively charged ions that attract each other. This bond is essential for the stability of compounds like table salt (NaCl), and it plays a key role in chemistry, biology, and material sciences.
What is an Ionic Bond?
An ionic bond is a type of chemical bond formed between two atoms when one atom donates electrons and another atom accepts them. Typically, this bond happens between metals and nonmetals:
-
Metals lose electrons, becoming positively charged cations.
-
Nonmetals gain electrons, becoming negatively charged anions.
For example:
-
Sodium (Na) donates one electron → Na⁺
-
Chlorine (Cl) accepts one electron → Cl⁻
-
Together, they form sodium chloride (NaCl) through an ionic bond.
Characteristics
The ionic bond has distinct properties that make it unique from covalent or metallic bonds.
-
High Melting and Boiling Points
Ioniccompounds like NaCl and KCl require high energy to break apart due to the strong electrostatic forces. -
Solubility in Water
Many ionic compounds dissolve easily in water, releasing ions into the solution. -
Electrical Conductivity
In the solid state, ioniccompounds do not conduct electricity. However, in molten or aqueous states, they conduct electricity because free ions are available. -
Crystal Lattice Structure
Ionic bonds form a regular arrangement of ions called a crystal lattice, making the compounds hard and brittle. -
Brittleness
When force is applied, ionic compounds tend to shatter rather than bend, due to repulsion between like-charged ions.
Formation of Ionic Bond
The process of forming an ionic bond involves three main steps:
-
Electron Transfer – A metal atom loses electrons, and a nonmetal atom gains them.
-
Ion Formation – Cations and anions are formed with opposite charges.
-
Electrostatic Attraction – The opposite charges strongly attract each other, creating an ionicbond.
Example:
-
Magnesium (Mg) loses two electrons → Mg²⁺
-
Oxygen (O) gains two electrons → O²⁻
-
Together, they form magnesium oxide (MgO).
Examples of Ionic Compounds
Some common examples of compounds formed by ionic bonds include:
-
Sodium chloride (NaCl) – Table salt
-
Magnesium oxide (MgO) – Found in ceramics
-
Calcium carbonate (CaCO₃) – In chalk and shells
-
Potassium bromide (KBr) – Used in photography
Importance of Ionic Bond in Daily Life
The concept of ionic bond is not just limited to laboratories. It has real-world applications that impact everyday life:
-
Food – Sodium chloride (salt) enhances flavor and preserves food.
-
Medicine – Ionic compounds like potassium chloride and calcium supplements are essential for health.
-
Construction – Calcium carbonate and calcium oxide are vital in cement and concrete.
-
Electrolytes – Ionic compounds dissolved in water regulate nerve impulses and muscle functions in the human body.
Difference Between Ionic Bond and Covalent Bond
| Feature | Ionic Bond | Covalent Bond |
|---|---|---|
| Electron Sharing/Transfer | Transfer of electrons | Sharing of electrons |
| Bond Strength | Strong electrostatic forces | Relatively weaker forces |
| State at Room Temp | Solid (crystalline) | Can be solid, liquid, or gas |
| Conductivity | Conducts when molten or dissolved | Poor conductor |
Applications of Ionic Bond in Industries
-
Battery Production – Ionic compounds like lithium salts are essential in rechargeable batteries.
-
Glass and Ceramics – Magnesium and calcium oxides make strong and heat-resistant materials.
-
Water Treatment – Ionic compounds such as alum help in purification.
-
Pharmaceuticals – Drugs often use ionic bonding for stability and bioavailability.
Conclusion
The ionic bond is a cornerstone of chemistry that explains the stability of countless compounds. By transferring electrons, atoms form ions that attract strongly, resulting in hard, crystalline structures with unique physical and chemical properties. From the salt we consume to the batteries that power our devices, ionic bonds are everywhere around us. Understanding them helps us connect fundamental science with practical life.